Use the "Next" and "Back" buttons located in the corners to turn the pages of the book.
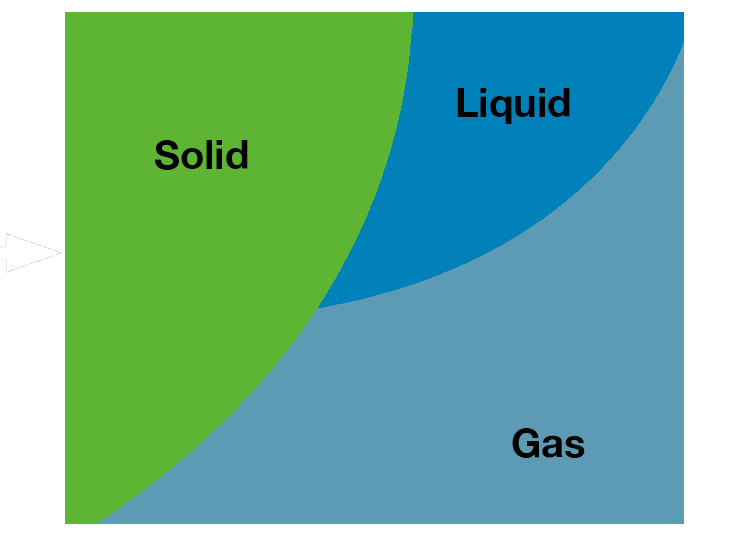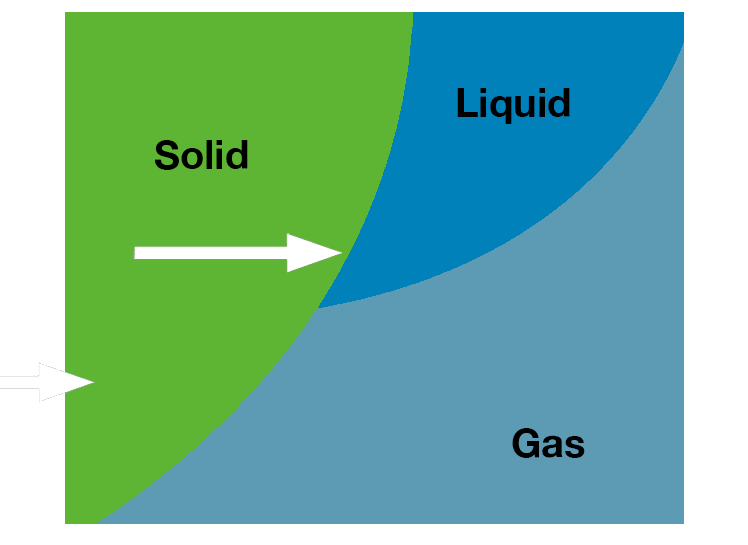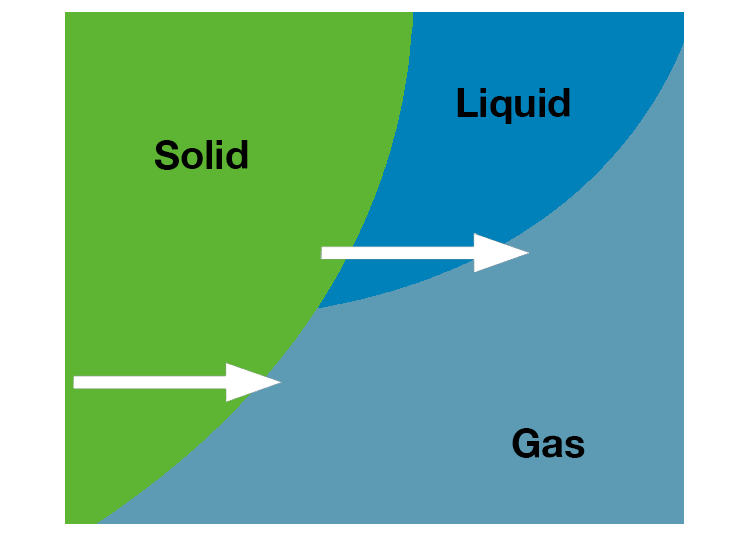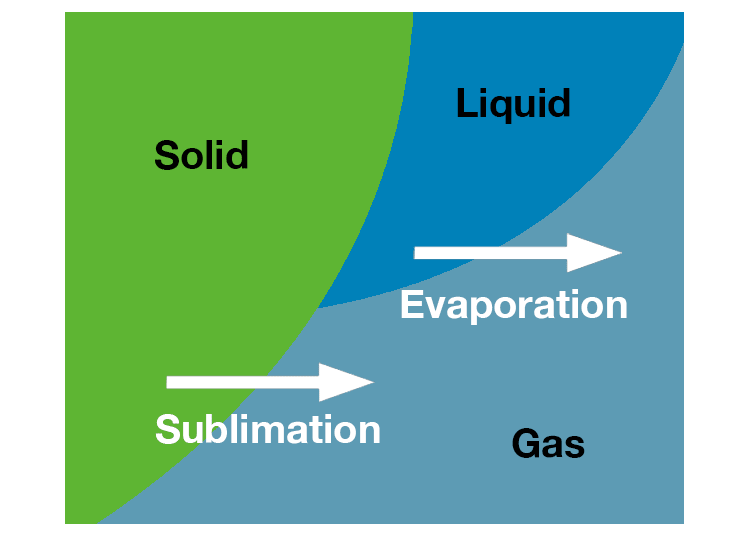


Concentration of samples utilizes evaporation, or converting liquid solvents into the vapor form. Lyophilization of samples utilizes sublimation, or converting solid, frozen solvents directly into the vapor form.
Bypassing the liquid phase gives samples excellent preservation properties and is one of the gentlest ways to dry a sample.


LYOPHILIZATION
- Freeze dryers use deep vacuum (<0.400 mbar) and heat to remove moisture from a sample
- Requires a frozen sample
- The unique phase change is called sublimation
- Ideal for heat sensitive samples

VACUUM CONCENTRATION & VACUUM EVAPORATION
- Utilize heat and vacuum to evaporate
- Centrifugal force is used to prevent sample bumping, a term used to describe samples aggressively boiling out, potentially causing cross-contamination
- Vacuum evaporation is better suited for a small number of high volume, volatile samples. Vacuum concentration is better suited for a large number of low volume samples
- Solvent recovery is possible with the use of a cold trap or glass trap



NITROGEN BLOW DOWN EVAPORATION
- Utilize heat and a nitrogen stream to evaporate
- Vortex motion within the sample increases surface area allowing for more rapid evaporation and solute washdown
- Allows for rapid evaporation of heat-tolerant samples


APPLICATION GUIDE
Aqueous, protein, peptide, vegetation, environmental, HPLC, pharmaceutical
Peptides, DNA, protein, oligonucleotides, pharmaceutical
Environmental, EPA, toxicology, biological, microbiology, food chemistry, pharmaceutical
FACT:
Temperature, pressure, and surface area each has a direct correlation on the rate of evaporation.
CENTRIFUGAL CONCENTRAION VS. NITROGEN EVAPORATION
Centrifugal Concentration
- Reduced pressure via vacuum pump
- Samples are held in a spinning rotor, creating a pressure gradient that allows samples to boil from the surface, preventing bumping
- Allows for solvent recovery
- Allows for concentration to a wetted pellet, or dryness
Nitrogen Evaporation
- Reduced partial pressure directly over sample
- Creates vortex motion within sample container to increase surface area
- The vortex motion continually washes down solutes for excellent analyte recovery
- Evaporate to dryness or a concentrated liquid